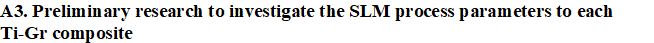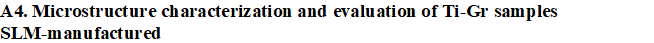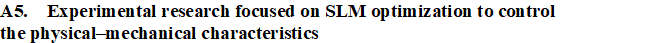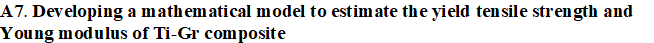English
New Additive Technology to produce Custom Medical Implants with Controlled physical-mechanical characteristics and antibacterial properties
(Acronym MediCo)
Objective
The main objective of the project is to develop a new technology that enables to produce customised maxillofacial implants with controlled physical-mechanical characteristics and antibacterial properties made of titanium-graphene composite powder by SLM manufacturing.
The specific objectives of the project are include in the following Working Plans (WP):
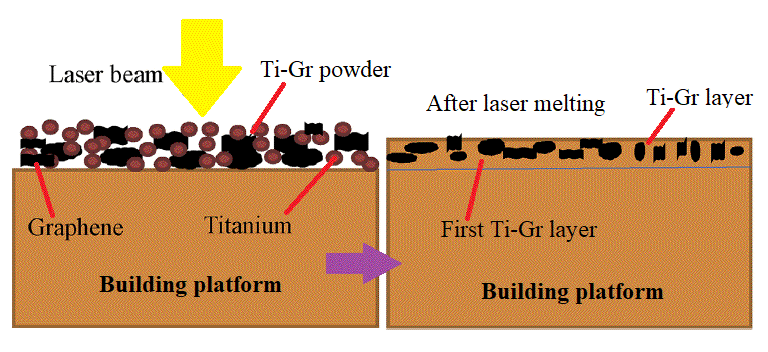
 WP 1. Develope new composites for SLM: This approach presumes preparing mixed Ti-Gr composite powders adapted to SLM manufacturing in various compositions of graphene. Preliminary experiments will analysis the SLM process parameters to establish their range and microstructure characterization will be developed (XRD, SEM, and EDAX).
WP 1. Develope new composites for SLM: This approach presumes preparing mixed Ti-Gr composite powders adapted to SLM manufacturing in various compositions of graphene. Preliminary experiments will analysis the SLM process parameters to establish their range and microstructure characterization will be developed (XRD, SEM, and EDAX). WP 2. Optimize the SLM technology for new Ti-Gr composite: The experimental research will improve the SLM manufacturing to fabricate Ti-Gr specimens with controlled physical–mechanical characteristics. Using Design-Expert and Analysis of Variance (ANOVA), a regression model will be empirical developed to optimize and estimate the SLM process parameters for a specified yield strength and Young modulus. The predicted SLM parameters by the regression equation will be tested and validated. Mutiple Ti-Gr specimens will be SLM manufactured with different sets of laser parameters and physical–mechanical tested.
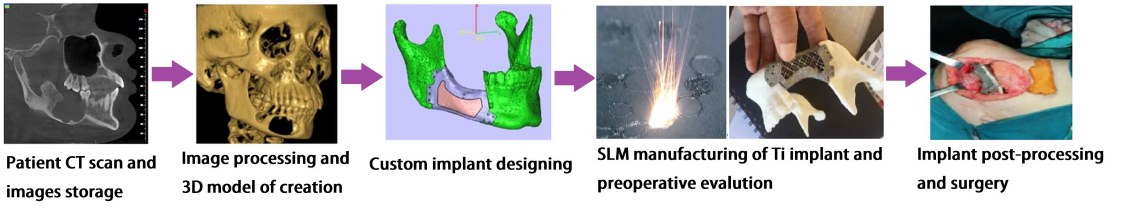
WP 2. Optimize the SLM technology for new Ti-Gr composite: The experimental research will improve the SLM manufacturing to fabricate Ti-Gr specimens with controlled physical–mechanical characteristics. Using Design-Expert and Analysis of Variance (ANOVA), a regression model will be empirical developed to optimize and estimate the SLM process parameters for a specified yield strength and Young modulus. The predicted SLM parameters by the regression equation will be tested and validated. Mutiple Ti-Gr specimens will be SLM manufactured with different sets of laser parameters and physical–mechanical tested. WP 3. Validate the SLM technology for Ti-Gr and disseminating the results: Starting from computed tomography (CT) images, customized maxillofacial implants will be designed. Knowing the physical-mechanical properties of Ti-Gr and bone tissue, the implant biomechanics will be simulated and evaluated. FEA study will help to expand the concept of implant customization from structural and mechanical point of view, according to patient’s age and health status. Controlling the physical–mechanical characteristics of Ti-Gr implants (e.g., Young modulus), a suitable balance between strength and stiffness can be found. Also, the implant weight can be reduced via toplogical surface optimization (tensile strength of Ti-Gr will be superior to conventional pure Ti). To test and validate this new AM technology, various complex and anatomical maxillofacial implants will be SLM manufactured from Ti-Gr composite and physical tests (compression and shock).
WP 3. Validate the SLM technology for Ti-Gr and disseminating the results: Starting from computed tomography (CT) images, customized maxillofacial implants will be designed. Knowing the physical-mechanical properties of Ti-Gr and bone tissue, the implant biomechanics will be simulated and evaluated. FEA study will help to expand the concept of implant customization from structural and mechanical point of view, according to patient’s age and health status. Controlling the physical–mechanical characteristics of Ti-Gr implants (e.g., Young modulus), a suitable balance between strength and stiffness can be found. Also, the implant weight can be reduced via toplogical surface optimization (tensile strength of Ti-Gr will be superior to conventional pure Ti). To test and validate this new AM technology, various complex and anatomical maxillofacial implants will be SLM manufactured from Ti-Gr composite and physical tests (compression and shock). Activities:
Results:
- 4 mixed Ti-Gr powders with different concentration of graphene and values of flow rate.
- 40 sets of SLM process parameters for mixed Ti-Gr powders.
- Over 80 samples SLM-manufactured with different parameters.
- SEM images and EDAX analyses.
- XRD diffraction of Ti-Gr composite samples SLM-manufactured.
- 1 mathematical model developed for Ti-Gr used to estimate mechanical properties.
- 5 sets of SLM parameters configured via mathematical regression.
- Physical-mechanical properties of Ti-Gr samples SLM-processed.
- 3 virtual models of maxillo-facial implants.
- FEA results focused on biomechanical behavior of Ti-Gr implants.
- 5 maxillo-facial implants SLM-manufactured using Ti-Gr powder and mechanical tested.
Manner of disseminating the results:
- 2 scientific papers published in ISI journals;
- 2 scientific papers published in ISI or BDI journals;
- 1 patent request;
- 4 bachelor theses in project topic;
- 1 workshop with national and international participants.